Calculation of the dissociation constant of glycoprotein
Calculation of the dissociation constant of glycoprotein
The calculation of the dissociation constant of glycoprotein and lectin is explained as follows. First, asialofetuin (glycoprotein) was immobilized as ligand on a sensor. Then, concanavalin A (lectin) was injected and the reaction speed and reaction amount were measured. By using "NAPiCOS analysis (analysis software attached to NAPiCOS)", the dissociation constant was calculated.
1.Protocol
- Sensor:30MHz twin sensor
- Flow rate:50μL/min
- Sample amount:100μL
- Running buffer:PBS
- Sample:Concanavalin A
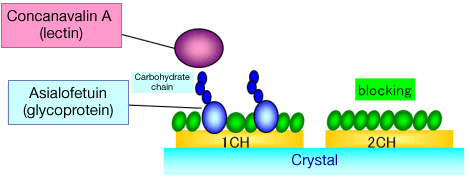
Fig.1: Diagram
2.Reaction waveform
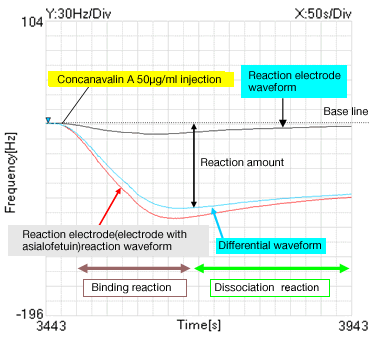
Fig.2: Binding & dissociation waveform
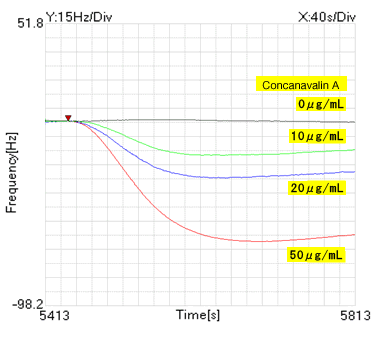
Fig.3: Differential waveform after concanavalin A injection
3.Calculation of dissociation constant
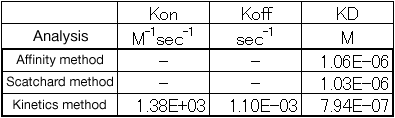
By using our analysis application "NAPiCOS analysis", the dissociation constant and the rate constant for reaction were calculated based on the reaction amount of concanavalin A (concentration: 10 μg/mL, 20 μg/mL, 50 μg/mL). The table below shows the dissociation constant and rate constant for reaction calculated with 3 kinds of analysis methods. The rate constant for reaction can be calculated only when the Kinetics method is used.
4.Glossary
| Asialofetuin | A protein obtained by removing sialic acid from a glycoprotein called fetuin. The molecular weight is 40,000. |
| Concanavalin A | A lectin (sugar chain-binding protein) that agglutinates red blood cells by binding to the sugar chain on the surface of red blood cells.The molecular weight is 100,000. |
| Dissociation constant | The ratio between the concentration of the reacting substances and product in equilibrium. This is used as the gauge of the affinity between substances. |
| Rate constant for reaction | The rate of increase or decrease of reacting substances or products. |
